IAIP Biomarker
We have developed a new, highly specific and sensitive assay to measure blood IAIP levels and have determined that IAIP is a unique, predictive biomarker for severe inflammatory diseases.
Since IAIP levels are high in healthy individual’s blood, and significantly decrease during severe inflammation, and the levels correlate inversely with the disease severity, an IAIP blood test is clinically important as a biomarker to identify progression to acute, life- threatening diseases.
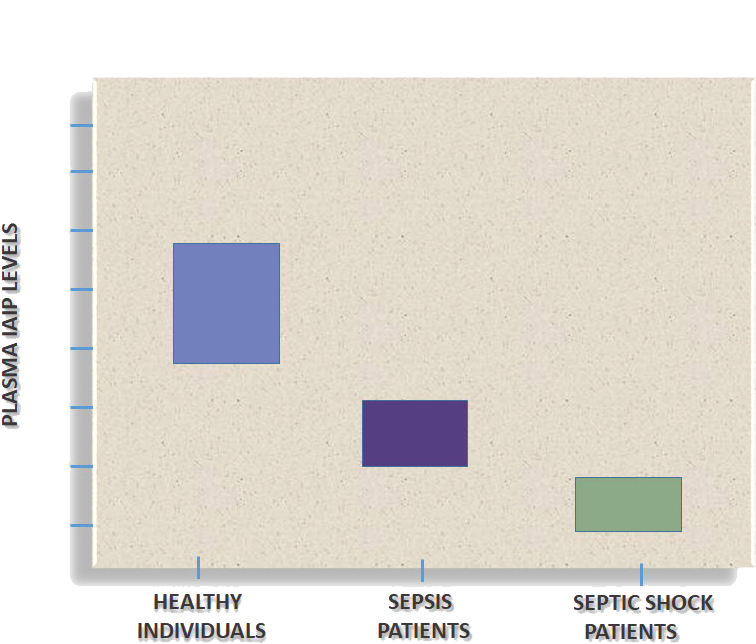
Low levels of IAIP signal poor outcomes for patients
Thousands of patient blood samples have been evaluated to support the involvement of IAIP in the host inflammatory response and form a strong basis for the biomarker applications.
We are translating our biomarker discovery into a new bedside Rapid Test to provide physicians with potentially life-saving information about the patient’s level of inflammation.
An IAIP, hand held, Rapid Test will enable physicians to determine the course of disease at the bedside and quickly identify patients at risk. Using a drop of patient’s blood, the test will also allow physicians to monitor the response to therapies “real time” and to monitor blood levels of IAIP during replacement therapy.
With funding from the National Institutes of Health, we are developing the test to assess the risk of infant patients for developing sepsis or necrotizing enterocolitis (NEC), a serious gastrointestinal disease. Infants can display non specific symptoms and there is currently no method to determine if these patients will progress to life-threating inflammatory conditions.
The IAIP Rapid Test is under development to fill this unmet medical need.
Our goal is to save the lives of infants.
Inter-alpha Inhibitor Proteins have the advantage of being both a biomarker and a therapeutic
